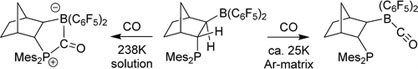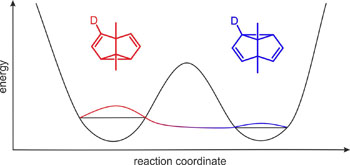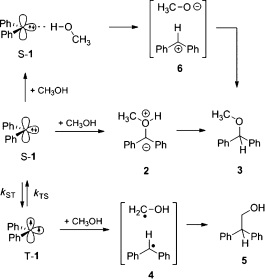
Paolo Costa won the Dr.-Heinrich-Kost-Preis of the Ruhr University for his PhD thesis
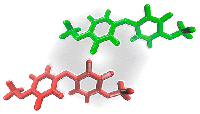
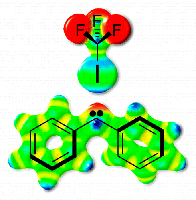

 The Highly Reactive Benzhydryl Cation Isolated and Stabilized in Water Ice
The Highly Reactive Benzhydryl Cation Isolated and Stabilized in Water Ice
P. Costa, M. Fernandez-Oliva, E. Sanchez-Garcia, W. Sander, J. Am. Chem. Soc. 136 (2014), 15625-15630.
Read our publication in J. Am. Chem. Soc.
More about our Research on Carbenes