Research Interests
Research projects in the Neumann Group involve the exploration of new targets for antibiotics and the development of methods that improve antibiotic delivery, and combine fundamental characterization of naturally occurring metal chelators with molecular design and application for antibacterial strategies.
Transition metal ions (e.g., Mn, Fe, Zn) are essential nutrients for all organisms and play important roles in cells. They function as electron transfer centers and cofactors that catalyze chemical transformations, and fulfill structural and regulatory functions. Bacteria must acquire these metal nutrients from the environment, rendering them important players at the host/pathogen interface. To successfully colonize the human host, bacteria utilize various sophisticated metal acquisition systems that include the production and secretion of low-molecular-weight metal chelators, termed “metallophores” (or “siderophores” if they function as ferric iron chelators). After metal binding, the formed complexes are taken up through dedicated membrane transporters, and the bound metal ions are released intracellularly. To counteract pathogenic invaders, the human host mounts different mechanisms that affect bacterial metal homeostasis, including a metal-withholding immune response that starves microbes of essential nutrients, and an immune response that intoxicates invaders with high concentrations of toxic metal ions.
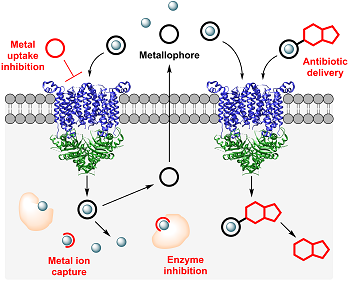
Although acquisition of sufficient nutrient metal ions is crucial, metal dysbalance can result in serious cell damage caused by mismetallation or formation of reactive oxygen species, rendering both the bacterial metal uptake systems and metallome valuable targets for antibacterial strategies. We investigate natural products that can perturb the bacterial metallome or inhibit metalloenzymes. Moreover, we investigate bacterial metallophore systems and how metal ion uptake can be inhibited. We also exploit the bacterial metal uptake machinery for the targeted delivery of antibiotics. By attaching antibiotics to metallophores, the low permeability of bacterial cell membranes, which impedes antibiotic uptake and considerably contributes to antibiotic resistance, can be overcome. Moreover, this approach allows for a pathogen-specific delivery of antibiotics, thereby limiting the spreading of antibiotic resistance and preserving the commensal microbiota of the host.